Praxis Precision Medicines focuses on Essential Tremor and Epilepsy. They have no FDA approved drug and a weak pipeline. Their primary drug Ulixacaltamide will very likely fail in phase 3. They have high negative FCF and dillute their stock very much. Their only advantage is having a decent cash position with zero debt. The stock sank very much the last months but unless they announce something very positive the stock might sink even more.
Balance Sheet
Q1 25. Units in million except price and runway.
| price | 37.2 |
| mc | 750 |
| cash | 327 |
| debt | 1 |
| ev | 424 |
| shares | 21 |
| aPIC | 1,344 |
| ad | 906 |
Income Statement
Units in million.
| 2024 | 2023 | |
|---|---|---|
| rev (collabartion) | 9 | 2 |
| rnd | 152 | 86 |
| ga | 56 | 42 |
| net loss | -182 | -123 |
| FCF | -132 | -111 |
| shares | 18 | 7 |
Pipeline
| pipeline | phase | indication | moa | catalyst |
|---|---|---|---|---|
| Ulixacaltamide | 3 | Essential Tremor | T-type calcium channel modulator | Topline data Q3 2025 |
| Vormatrigine | 2/3 | Focal & Generalized Epilepsy | Sodium channel state modulator | RADIANT data H1 2025, POWER1 H2 2025 |
| Relutrigine | 2 | Pediatric DEE Epilepsies | Sodium channel state modulator | Cohort 2 data H1 2026 |
| Elsunersen | Pre-Registration | SCN2A GoF DEE | Gapmer ASO | Pivotal trial start H1 2025 |
| PRAX-020 | Preclinical | KCNT1 Epilepsy | KCNT1 specific inhibitor | Licensed to UCB |
| PRAX-080 | Preclinical | PCDH19 Mosaic Epilepsy | Gapmer ASO | Early-stage discovery |
| PRAX-090 | Preclinical | SYNGAP1 LoF | Splice switching ASO | Preclinical development |
| PRAX-100 | Preclinical | SCN2A LoF | Undisclosed mechanism ASO | Preclinical development |
Ulixacaltamide
is in CT III with topline results expected in Q3 25. IDMC indicated that the trial is unlikely to meet its primary efficacy endpoint. Stock fell 40% by this anouncement. Suvecaltamide by Jazz Pharmaceuticals was also tested for essential tremor and did not meet primary endpoint.
Lipinski Rule of 5
| Property | Value |
|---|---|
| MW | 383.18 |
| HBA | 3 |
| HBD | 2 |
| RB | 5 |
| LogP | 2.84 |
Description
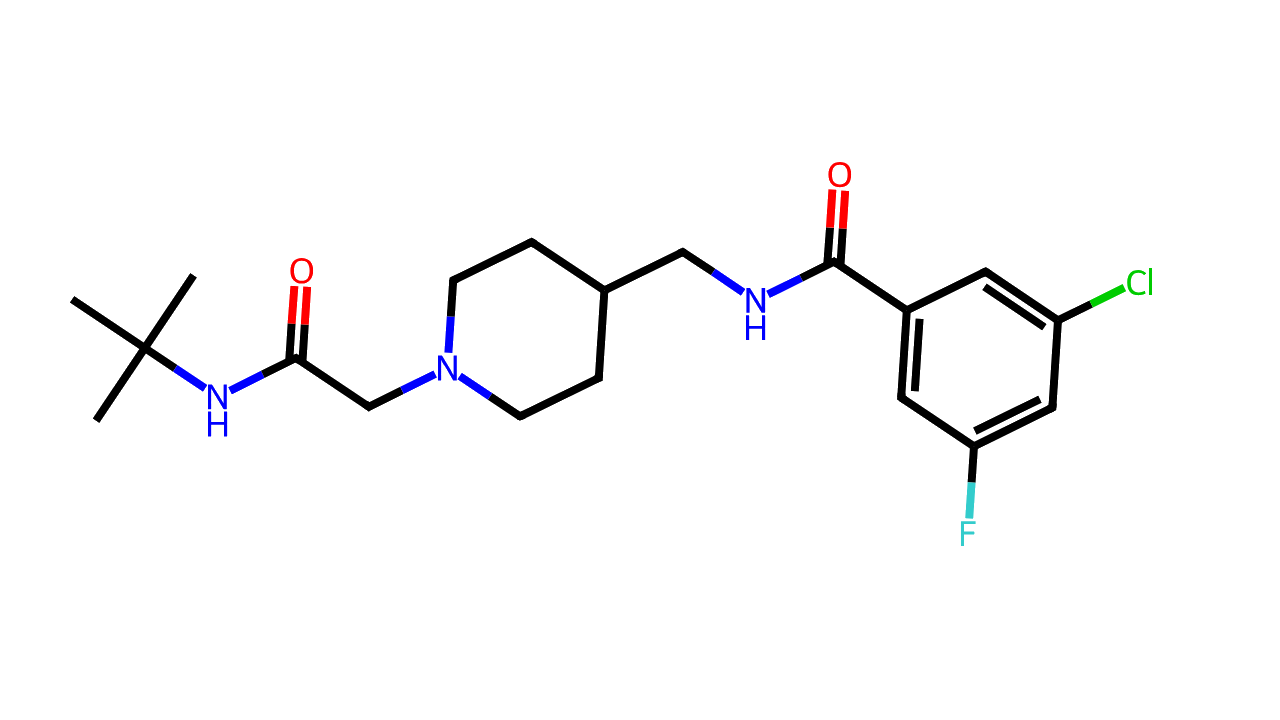
Ulixacaltamide (Prax-944, Z944) is an investigational T-type calcium channel modulator being developed for the treatment of essential tremor.
N-((1-(2-(tert-ButylaMino)-2-oxoethyl)piperidin-4-yl)Methyl)-3-chloro-5-fluorobenzaMide
PD
selective T-type Ca2+ ion channel blocker with IC50 values are 50 to 160 nM for hCav3.1, hCav3.2 and hCav3.3. Phase I trial on healthy humans showed significant reductions in sigma-band power (11–15 Hz) during non-REM sleep.
PK
not publicly available so this is an example from another drug:
Following oral dose, plasma peak concentration is approximately 6 h. About 40% and is eliminated through first pass metabolism. The half-life is from 21 to 54 h and plasma clearance is from 12 to 47 h. Daily administration will lead to steady state of plasma concentration in about a week with concentration twice that of the single dose. Metabolism of Zyprexa is by the cytochrome P450 oxidation
Toxicity
At 30 times the maximum human dose, rats led to reductions in locomotor activity, with significant motor side effects observed only at supratherapeutic doses. Humans received up to 120 mg/day with mild TEAE like headache, dizziness, fatigue, anxiety.